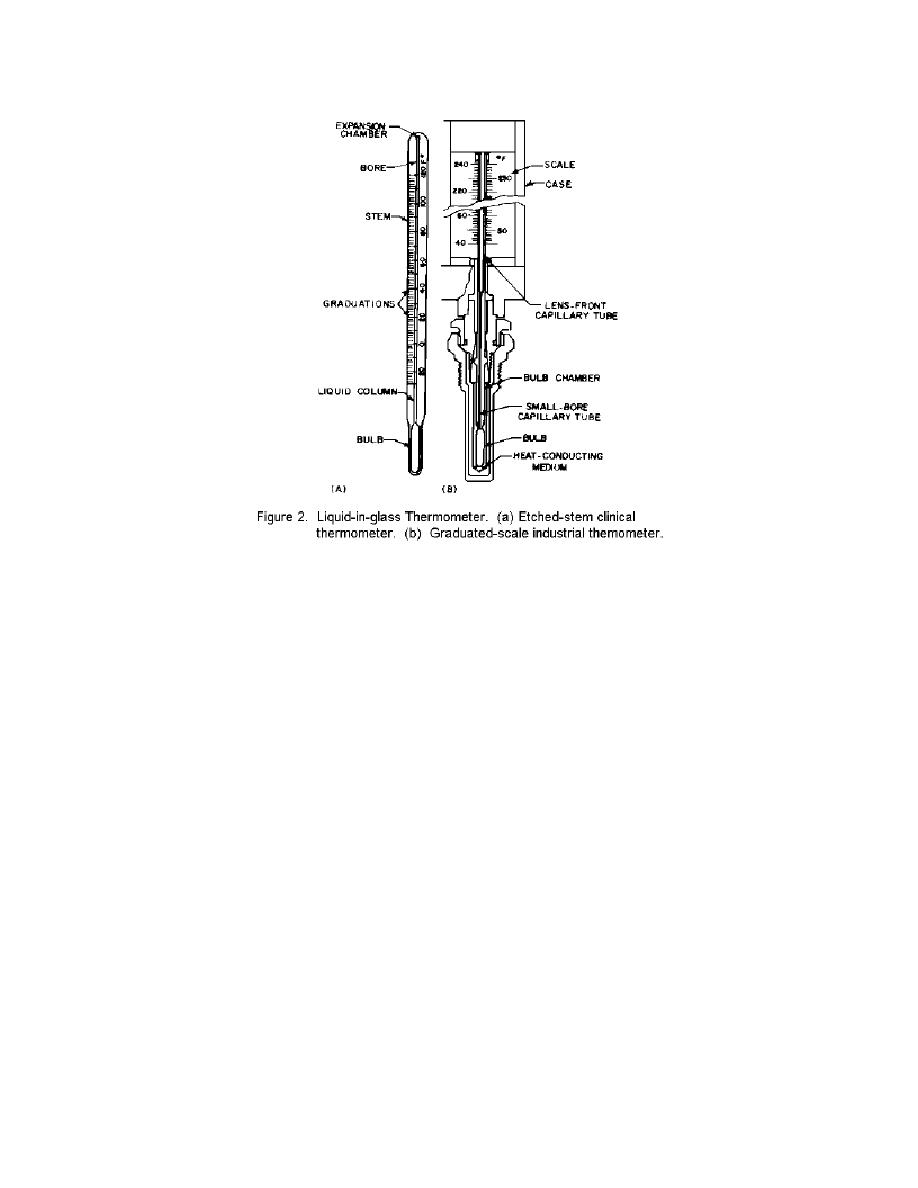
Sensitivity to a rising temperature causes both the glass and mercury to expand.
However, the mercury expands more than the glass, forcing mercury up the capillary. As
you observe the mercury column, it is very practical to etch the temperature scale on the
glass tube. You no doubt have had your body temperature taken with a medical (mercury)
thermometer. If you were observant you should have noticed that the nurse shook the
thermometer. This was done to force the mercury down into the bulb so that an accurate
measurement could be made. The mercury in some thermometers will separate. A
thermometer in this condition can not be used. The mercury must be re-combined. One
way of doing this is to heat the thermometer causing the mercury to expand up the
capillary tube and re-combine with any mercury that has separated. If the mercury will
not re-combine you must not use the thermometer. It is quite obvious that when the
mercury expands it has only one place to go (up the capillary tube).
The rate at which the mercury column rises is dependent on temperature change, but it is
also controlled by the diameter of the capillary tube. The accuracy of the mercury
thermometer depends on the uniformity of the glass reservoir and capillary tube.
Calibration of the temperature scale is also important.
The accuracy of the mercury thermometer is also dependent on the purity of the mercury.
If impurities are in the mercury, the expansion rate could be affected and the resultant
nonlinearity would produce erroneous readings.
NOTE: Do not confuse the mercury thermometer with the so-called thermometers you
find in 5 and 10 cent stores that contain red dye in an alcohol base.
4. IMMERSION SET.
Just as the freezing and boiling points of water restrict the range of the air thermometer,
mercury, which freezes at -38.87,C (Celsius) and boils at 356.58,C limits the range of
the mercury thermometer. The glass tubing is not critical as it melts a 500,C to 800,C.



 Previous Page
Previous Page
