SM0486
ment in the list to give a better explanation of what heat is.
Heat is a name
given to that form of energy which represents the total kinetic energy (force
created by molecular motion) possessed by the moving molecules of a body.
There
are other definitions for heat, but this one contains the concept you need to fully
understand the nature of heat.
(7) Heat and Energy. You have already learned something of the statues of
matter and energy, the basic relationship of energy to heat, and how energy is
transformed from one type to another (such as electrical energy to heat energy).
Our primary concern in this section of the lesson is to increase this knowledge to
the extent that you can:
(a) Visualize, understand, and describe the molecular theory of matter.
(b) Understand the
relationship
between
the
molecular
structure
of
matter and kinetic energy.
(c) Understand the relationship between kinetic energy and heat.
(d) Associate the forms of energy and the transformation of energy with
heat.
(e) Apply your knowledge of the molecular structure of matter, the
relationship of molecular activity to kinetic energy, and the relationship of
kinetic energy and heat to the heat measurements you make.
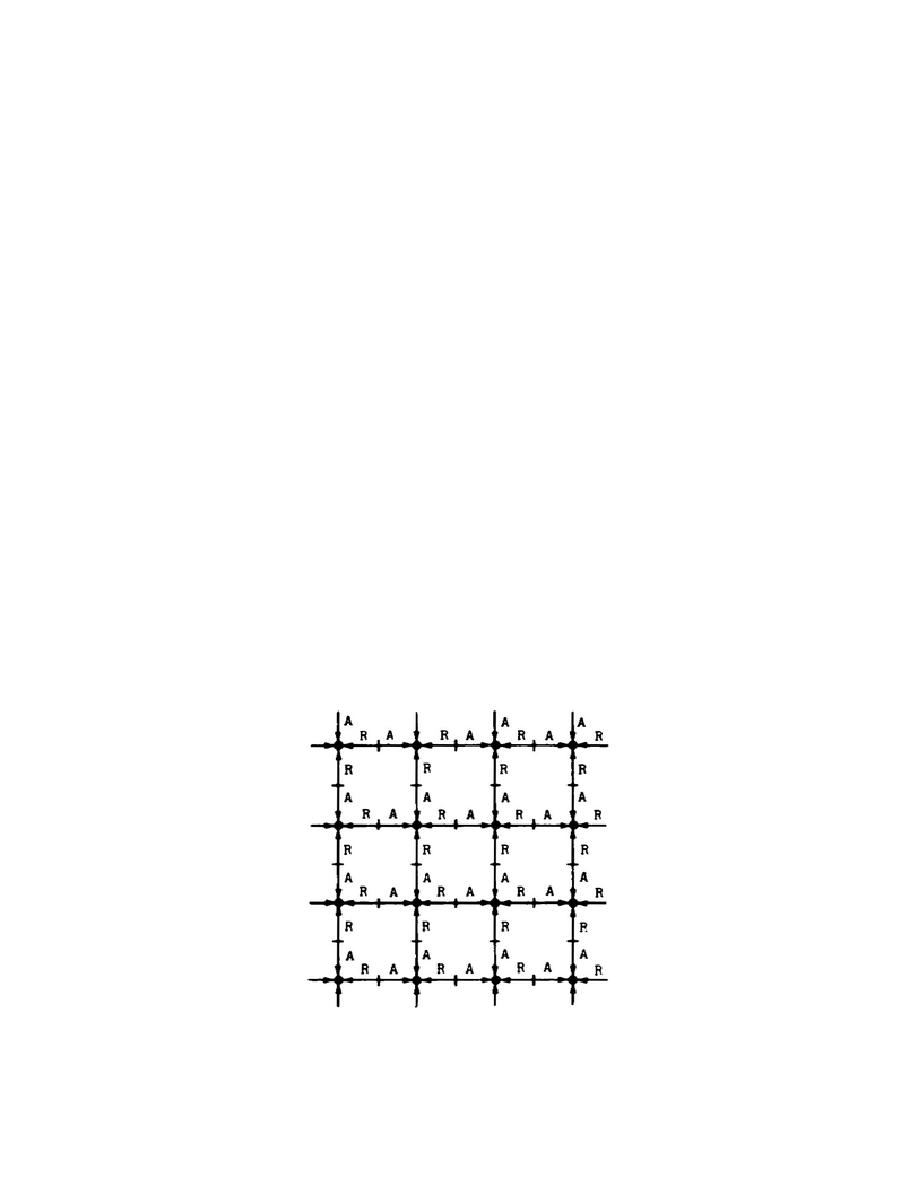
c. The molecular theory of matter. Let's assume that all matter is composed
of tiny particles called molecules and the molecules are arranged in a lattice
structure, as shown in figure 1. The individual molecules attract or repel their
neighbors in accordance with the separation between molecules. Generally speaking,
when the separation is large, the force between molecules is small and is one of
attraction. The molecules of the material represented in figure 1 are located at
separations such that the forces of attraction and repulsion are equal to support
our discussion.
Figure 1.
Molecular lattice structure.
3



 Previous Page
Previous Page
