SM0486
(1) The letters R and A shown in figure 1 represent the forces of repulsion
(R) and attraction (A) between the molecules. The line drawn through the middle of
the lines between molecules is used to show that the forces of repulsion and
attraction are equal to that distance. When a fixed lattice as shown (figure 1),
the forces between molecules nearly cancel each other so that there is very little
vibratory motion.
(2) The lattice structure shown in figure 1 is similar to the symmetry of
structure in crystals. If the molecules are pressed closer together, the force of
repulsion increases.
If the molecules are forced farther apart, the force of
attraction increases. External forces exerted on the molecules of the lattice in a
solid cause the molecules to vibrate about their center positions. This vibration
motion is relatively weak, and the centers of the molecules remain fixed.
(3) In liquids the molecules are free to move greater distances. Since the
vibratory motion in liquids is greater than in solids, the energy which the moving
molecules can transfer to other molecules (kinetic energy) is greater.
The
relationships between molecular separation forces, molecular kinetic energy, and
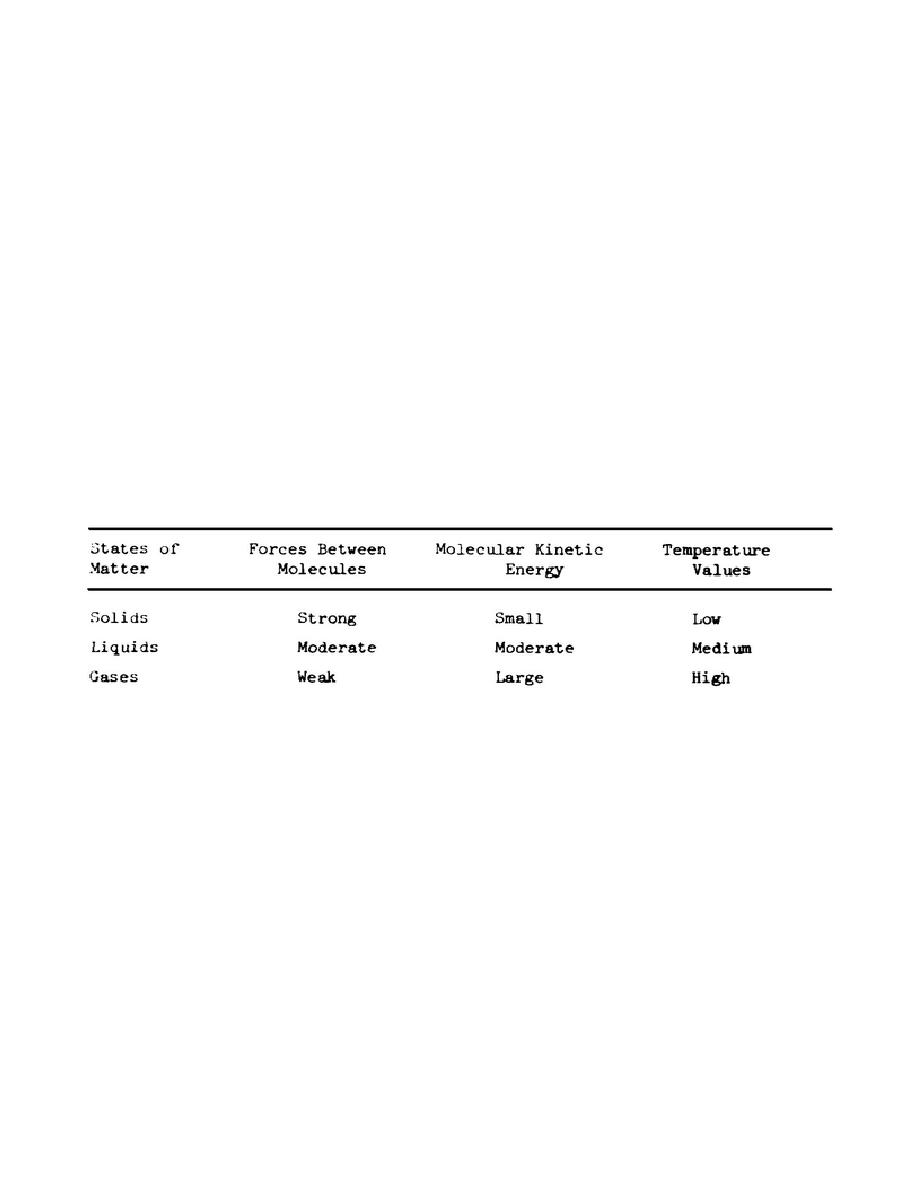
resulting-temperature conditions are shown in Table 1.
TABLE 1
THERMAL PROPERTIES OF SOLIDS, LIQUIDS, AND GASES
d. From the preceding paragraphs and Table 1, you should conclude that:
(1)
Heat is the kinetic energy which a body possesses.
(2)
Heat can be generated by means of electricity,
compression,
or
(3)
Kinetic energy is the work potential which a body possesses because of
its motion.
(4)
Normally, the forces of attraction between molecules in a solid are
strong, the
molecular energy is small, and the temperature values are low.
e. Molecular kinetic-energy level changes.
Let us restate our conclusions
from the preceding paragraphs in a simple statement.
When we think of heat, we
should think of kinetic energy. Because molecular kinetic
4



 Previous Page
Previous Page
